Visible-light-promoted synthesis of phenanthridines via an intermolecular isocyanide insertion reaction†
Abstract
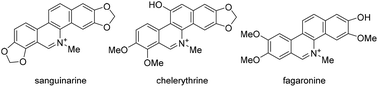
An isocyanide insertion reaction promoted by the combination of an amide and a photoredox is now presented. By using visible light, 6-amidophenanthridine derivatives can be prepared in good chemical yields under mild and eco-friendly reaction conditions. The reaction was shown to proceed through an electron transfer sequence by mechanistic experiments, and thus enabled the efficient production of 24 compounds, each with one new intramolecular aromatic ring obtained. The synthesized compounds were tested to embody good antitumor, antimicrobial and neuraminidase inhibitory activities.

 Please wait while we load your content...
Please wait while we load your content...