Stereoselective synthesis of (−)-desethyleburnamonine, (−)-vindeburnol and (−)-3-epitacamonine: observation of a substrate dependent diastereoselectivity reversal of an aldol reaction†
Abstract
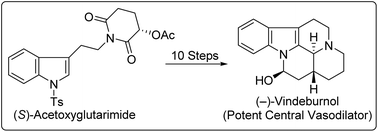
Starting from (−)-acetoxyglutarimide, the enantioselective multistep synthesis of (−)-desethyleburnamonine, (−)-vindeburnol and (−)-3-epitacamonine has been demonstrated via a common hydroxyl-lactam intermediate with very good overall yields. The acetoxy function from (−)-acetoxyglutarimide was initially used as a handle to induce enantioselectivity and then as a latent source of the ketone carbonyl group. Most importantly, substrate dependent reversal of the diastereoselectivity in ester aldol reactions of hexahydroindolo[2,3-a]quinolizinones has been reported.

 Please wait while we load your content...
Please wait while we load your content...