Synthesis and biological evaluation of palmyrolide A macrocycles as sodium channel blockers towards neuroprotection†
Abstract
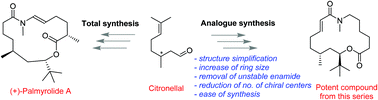
Palmyrolide A is a neuroprotective macrolide isolated by Gerwick and coworkers in 2010. This natural product is known to suppress neuronal spontaneous calcium ion oscillations through its voltage-gated sodium channel blocking ability which is of significant interest in CNS drug discovery. Herein, we give a detailed account on total synthesis of (+)-palmyrolide A and synthesis of a focused library of macrocycles around the scaffold, followed by their biological evaluation. Use of the chiral pool approach, Zhu's oxidative homologation, access to unnatural cis-palmyrolide A, preparation of 18 new analogues and identification of macrolides with improved sodium channel blocking activity are the important features of the present paper. As a measure of potency as voltage-gated sodium channel blockers, all the synthesized analogues were profiled for their ability to inhibit the veratridine-stimulated Na+ influx in murine primary neuronal cultures. Four macrocycles were found to be more potent or comparable to that of the natural product (−)-palmyrolide A. The most potent compound from this series 20 was structurally simplified and readily accessible in good quantities for further biological profiling.

 Please wait while we load your content...
Please wait while we load your content...