Insights into the structural patterns of the antileishmanial activity of bi- and tricyclic N-heterocycles†
Abstract
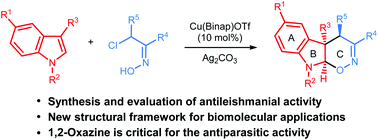
The influence of various structural patterns in a series of novel bi- and tricyclic N-heterocycles on the activity against Leishmania major and Leishmania panamensis has been studied and compounds that are active in the low micromolar region have been identified. Both quinolines and tetrahydrooxazinoindoles (TOI) proved to have significant antileishmanial activities, while substituted indoles were inactive. We have also showed that a chloroquine analogue induces Leishmania killing by modulating macrophage activation.

 Please wait while we load your content...
Please wait while we load your content...