New azo-decorated N-pyrrolidinylthiazoles: synthesis, properties and an unexpected remote substituent effect transmission†
Abstract
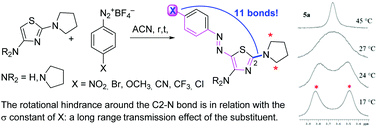
New 5-azo substituted thiazole derivatives have been obtained, under mild conditions and in good yields, by the reaction between 2-N-pyrrolidinylthiazole and a series of aryldiazonium salts bearing, mainly in the para position, groups with different electronic effects. The NMR spectra of the products show broad signals of the methylene groups in the alpha position to the pyrrolidinyl nitrogen, suggesting a hindered rotation around the C2–N bond, of which a double bond character was also evidenced by X-ray diffraction analyses. The free energies of activation for the rotational processes have been obtained from 1H NMR experiments and computer simulations at different temperatures and provided good correlation with the σ constants of the substituents on the ‘remote’ benzene ring. This represents an unexpected and peculiar result since the restricted rotation around the C2–N bond was shown to be influenced by a substituent situated very far away. 2,4-Di-N-pyrrolidinylthiazole showed a much greater reactivity than 2-N-pyrrolidinylthiazole with aryldiazonium salts.

 Please wait while we load your content...
Please wait while we load your content...