New biphenyl iminium salt catalysts for highly enantioselective asymmetric epoxidation: role of additional substitution and dihedral angle†
Abstract
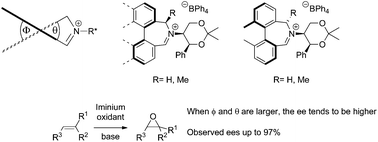
New biaryl iminium salt catalysts for enantioselective alkene epoxidation containing additional substitution in the heterocyclic ring are reported. The effects upon conformation and enantioselectivity of this additional substitution, and the influence of dihedral angle in these systems, has been investigated using a synthetic approach supported by density functional theory. Enantioselectivities of up to 97% ee were observed.
 Please wait while we load your content...
Please wait while we load your content...