Marine AChE inhibitors isolated from Geodia barretti: natural compounds and their synthetic analogs†
Abstract
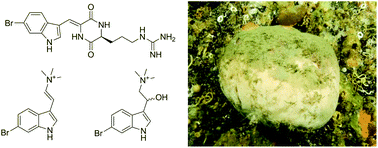
Barettin, 8,9-dihydrobarettin, bromoconicamin and a novel brominated marine indole were isolated from the boreal sponge Geodia barretti collected off the Norwegian coast. The compounds were evaluated as inhibitors of electric eel acetylcholinesterase. Barettin and 8,9-dihydrobarettin displayed significant inhibition of the enzyme, with inhibition constants (Ki) of 29 and 19 μM respectively towards acetylcholinesterase via a reversible noncompetitive mechanism. These activities are comparable to those of several other natural acetylcholinesterase inhibitors of marine origin. Bromoconicamin was less potent against acetylcholinesterase, and the novel compound was inactive. Based on the inhibitory activity, a library of 22 simplified synthetic analogs was designed and prepared to probe the role of the brominated indole, common to all the isolated compounds. From the structure–activity investigation it was shown that the brominated indole motif is not sufficient to generate a high acetylcholinesterase inhibitory activity, even when combined with natural cationic ligands for the acetylcholinesterase active site. The four natural compounds were also analysed for their butyrylcholinesterase inhibitory activity in addition and shown to display comparable activities. The study illustrates how both barettin and 8,9-dihydrobarettin display additional bioactivities which may help to explain their biological role in the producing organism. The findings also provide new insights into the structure–activity relationship of both natural and synthetic acetylcholinesterase inhibitors.

 Please wait while we load your content...
Please wait while we load your content...