A selective C–H insertion/olefination protocol for the synthesis of α-methylene-γ-butyrolactone natural products†
Abstract
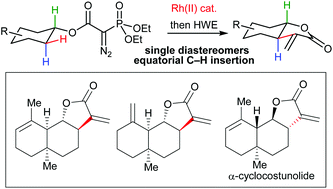
A regio- and stereoselective one-pot C–H insertion/olefination protocol has been developed for the late stage installation of α-methylene-γ-butyrolactones into conformationally restricted cyclohexanol-derivatives. The method has been successfully applied in the total synthesis of eudesmanolide natural product frameworks, including α-cyclocostunolide.

 Please wait while we load your content...
Please wait while we load your content...