Synthesis of dibenzylamino-1-methylcyclohexanol and dibenzylamino-1-trifluoromethylcyclohexanol isomers†
Abstract
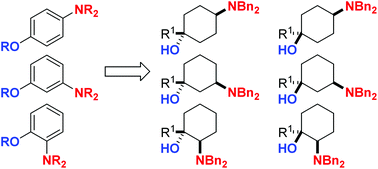
The isomers of dibenzylamino-1-methylcyclohexan-1-ol and dibenzylamino-1-trifluoromethylcyclohexan-1-ol have been prepared. The stereochemistry of these compounds was unequivocally assigned through a combination of NMR spectroscopy and single crystal X-ray analysis. The cis-isomer of 3-N,N-dibenzylamino-1-trifluoromethylcyclohexanol and its derivatives display an unusual conformational behaviour in both solution-phase and the solid-state, where the amino group usually adopts an axial conformation.

 Please wait while we load your content...
Please wait while we load your content...