The decrease in the IgG-binding capacity of intensively dry heated whey proteins is associated with intense Maillard reaction, structural changes of the proteins and formation of RAGE-ligands
Abstract
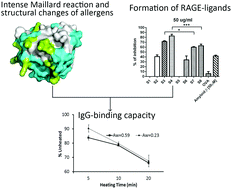
Heat treatment is the most common way of milk processing, inducing structural changes as well as chemical modifications in milk proteins. These modifications influence the immune-reactivity and allergenicity of milk proteins. This study shows the influence of dry heating on the solubility, particle size, loss of accessible thiol and amino groups, degree of Maillard reaction, IgG-binding capacity and binding to the receptor for advanced glycation end products (RAGE) of thermally treated and glycated whey proteins. A mixture of whey proteins and lactose was dry heated at 130 °C up to 20 min to mimic the baking process in two different water activities, 0.23 to mimic the heating in the dry state and 0.59 for the semi-dry state. The dry heating was accompanied by a loss of soluble proteins and an increase in the size of dissolved aggregates. Most of the Maillard reaction sites were found to be located in the reported conformational epitope area on whey proteins. Therefore the structural changes, including exposure of the SH group, SH–SS exchange, covalent cross-links and the loss of available lysine, subsequently resulted in a decreased IgG-binding capacity (up to 33%). The binding of glycation products to RAGE increased with the heating time, which was correlated with the stage of the Maillard reaction and the decrease in the IgG-binding capacity. The RAGE-binding capacity was higher in samples with a lower water activity (0.23). These results indicate that the intensive dry heating of whey proteins as it occurs during baking may be of importance to the immunological properties of allergens in cow's milk, both due to chemical modifications of the allergens and formation of AGEs.

 Please wait while we load your content...
Please wait while we load your content...