A Gibeon meteorite yields a high-performance water oxidation electrocatalyst†
Abstract
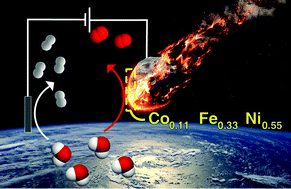
Examining the electrocatalytic performance of naturally-occurring metallic minerals is of interest for energy conversion applications given their unique atomic composition and formation history. Herein, we report the electrocatalytic function of an iron-based Gibeon meteorite for the oxygen evolution reaction (OER). After ageing under operational conditions in an alkaline electrolyte, an activity matching or possibly slightly superior to the best performing OER catalysts emerges, with stable overpotentials as low as 270 mV (for 10 mA cm−2) and Tafel slopes of 37 mV decade−1. The Faradaic efficiency for the OER was unity and no deterioration in performance was detected during 1000 hours of OER operation at 500 mA cm−2. Mechanistic studies suggest an operando surface modification involving the formation of a 3D oxy(hydroxide) layer with a metal atom composition of Co0.11Fe0.33Ni0.55, as indicated by Raman and XPS studies and trace Ir as indicated via elemental analysis. The growth of the catalyst layer was self-limiting to <200 nm after ca. 300 hours of operation as indicated through XPS depth profiling and cyclic voltammetry. The unique composition and structure of the Gibeon meteorite suggest that further investigation of Ir–Co–Ni–Fe systems or other alloys inspired by natural materials for water oxidation are of interest.

 Please wait while we load your content...
Please wait while we load your content...