A novel 3D Cu(i) coordination polymer based on Cu6Br2 and Cu2(CN)2 SBUs: in situ ligand formation and use as a naked-eye colorimetric sensor for NB and 2-NT†
Abstract
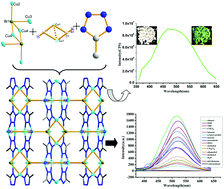
A novel coordination polymer with the chemical formula [Cu4Br(CN)(mtz)2]n (mtz = 5-methyl tetrazole) (1), has been synthesized under solvothermal conditions and characterized by elemental analysis, infrared (IR) spectroscopy, thermal gravimetric analysis, powder X-ray diffraction and single-crystal X-ray diffraction. Interestingly, the Cu(I), CN− and mtz− in compound 1 are all generated from an in situ translation of the original precursors: Cu2+, acetonitrile and 1-methyl-5-mercapto-1,2,3,4-tetrazole (Hmnt). The in situ ring-to-ring conversion of Hmnt into mtz− was found for the first time. Structural analysis reveals that compound 1 is a novel 3D tetrazole-based Cu(I) coordination polymer, containing both metal halide cluster Cu6Br2 and metal pseudohalide cluster Cu2(CN)2 secondary building units (SBUs), which shows an unprecedented (3,6,10)-connected topology. Notably, a pseudo-porphyrin structure with 16-membered rings constructed by four mtz− anions and four copper(I) ions was observed in compound 1. The fluorescence properties of compound 1 were investigated in the solid state and in various solvent emulsions, the results show that compound 1 is a highly sensitive naked-eye colorimetric sensor for NB and 2-NT (NB = nitrobenzene and 2-NT = 2-nitrotoluene).

 Please wait while we load your content...
Please wait while we load your content...