An accurate multi-channel multi-reference full-dimensional global potential energy surface for the lowest triplet state of H2O2
Abstract
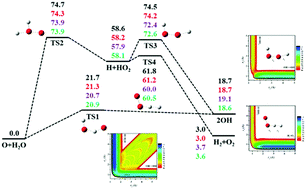
The lowest triplet state of the H2O2 system features multiple reaction channels, including several relevant to the combustion of H2. To accurately map out the global potential energy surface, ∼28 000 geometries were sampled over a large configuration space including all important asymptotes, and electronic energies at these points were calculated at the level of the explicitly correlated version of the multi-reference configuration interaction (MRCI-F12) method. A new multi-channel global potential energy surface was constructed by fitting the ab initio data set using a permutation invariant polynomial-neural network method, resulting in a total root mean square fitting error of only 6.7 meV (0.15 kcal mol−1). Various kinetics and dynamical properties of several relevant reactions were calculated on the new MRCI potential energy surface, and compared with the available experimental results.

 Please wait while we load your content...
Please wait while we load your content...