Formation of lamellar micelle-like oligomers and membrane disruption revealed by the study of short peptide hIAPP18–27†
Abstract
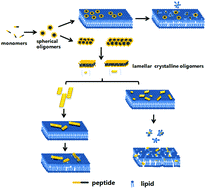
Prefibrillar amyloid aggregates of proteins are known as cytotoxic species and a common pathogenic factor for many degenerative diseases. The mechanism underlying the formation and cytotoxicity of prefibrillar aggregates is believed to be independent of the actual nature of the amyloid protein. In this study, we monitored the formation of the peptide oligomers and examined the disruptive effects of the oligomers on liposomes using the human islet amyloid polypeptide fragment hIAPP18–27 as a model peptide. We observed various morphologies of oligomers formed at different aggregation stages that precede the formation of mature amyloid fibrils. These oligomer species were sufficiently stable to maintain their structures and properties under acidic conditions. We presented the first evidence that an oligomer species with a lamellar crystalline structure and a size of about 20–60 nm in length, 8 nm in width and 1.5 nm in thickness was the most disruptive to the membrane containing the anionic component and toxic to the INS-1 cells. Our results showed that short peptides, in light of their slower fibrillation, could be used as a model system in the study of the toxic mechanism of misfolding oligomers of amyloid peptides.

 Please wait while we load your content...
Please wait while we load your content...