Janus head type lone pair–π–lone pair and S⋯F⋯S interactions in retaining hexafluorobenzene†
Abstract
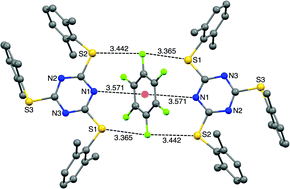
A series of eight tris-arylthiotriazines were synthesized to study the lone pair–π interaction between the triazine ring centroid of these molecules and halogenated solvents. All the eight compounds were characterized using 1H and 13C NMR spectroscopy and single crystal X-ray diffraction techniques. All these compounds show interesting structural properties in the solid state. Unprecedented Janus head type lp⋯π⋯lp and S⋯F⋯S interactions were observed between one of the tris-arylthiotriazines and hexafluorobenzene.
- This article is part of the themed collection: 2016 New talent

 Please wait while we load your content...
Please wait while we load your content...