On the electrochemical encounter between sodium and mesoporous anatase TiO2 as a Na-ion electrode†
Abstract
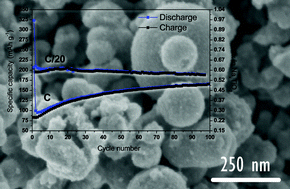
Mesoporous anatase titanium dioxide (TiO2) is prepared by an easily up-scalable synthesis protocol, using relatively inexpensive precursors. We demonstrate here that submicronic anatase TiO2 crystals show unexpected performances as electrodes of Na-ion batteries (NaBs). They exhibit highly stable reversible specific capacities of up to 200 mA h g−1 and excellent cyclability at moderate current rates at an average potential of 1.0 V vs. Na+/Na. While pseudocapacitance may appear to be the main process driving the reactions between the sodium ions and TiO2 during the first discharge above 1 V vs. Na+/Na, operando Raman and X-ray diffraction studies show that the TiO2 anatase structure is nearly entirely lost below 0.25 V vs. Na+/Na. The subsequent cycling is based on amorphous sodium titanate materials.
- This article is part of the themed collection: 2016 New talent


 Please wait while we load your content...
Please wait while we load your content...