Synthesis of tertiary arylamines: Lewis acid-catalyzed direct reductive N-alkylation of secondary amines with ketones through an alternative pathway†
Abstract
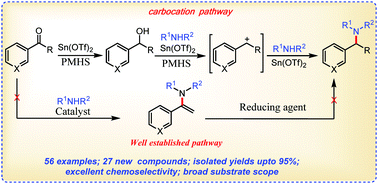
We report herein a highly efficient, tin(II)/PMHS catalyzed reductive N-alkylation of arylamines with ketones affording tertiary arylamines. A very wide substrate scope was observed for the current catalytic method as all six permutations of ketones/aldehydes/heterocyclic carbonyls and primary/secondary/heterocyclic amines were well tolerated, enabling access to secondary, tertiary and heterocyclic amines. The method is also convenient for the synthesis of N-substituted isoindolinones and phthalazinones via a tandem amination–amidation sequence. Mechanistic investigations revealed a carbocationic pathway instead of an ordinary direct reductive amination pathway.

 Please wait while we load your content...
Please wait while we load your content...