The catalytic enantioselective synthesis of tetrahydroquinolines containing all-carbon quaternary stereocenters via the formation of aza-ortho-xylylene with 1,2-dihydroquinoline as a precursor†
Abstract
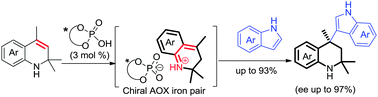
Tetrahydroquinolines (THQs) with an all-carbon quaternary stereocenter were effectively obtained via the in situ formation of aza-ortho-xylylene (AOX) with easily accessible 1,2-dihydroquinolines as precursors. The reaction was rationalized with chiral phosporic acid to afford chiral THQs with high yield and excellent enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...