Proton transfer in acetaldehyde–water clusters mediated by a single water molecule†
Abstract
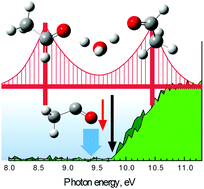
Proton transfer in aqueous media is a ubiquitous process, occurring in acid–base chemistry, biology, and in atmospheric photochemistry. Photoionization mass spectrometry coupled with theoretical calculations demonstrate that a relay-type proton transfer mechanism is operational for single-water-molecule-assisted proton transfer between two acetaldehyde molecules in the gas phase. Threshold photoionization of acetaldehyde–water clusters leads to proton transfer between the formyl groups (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of one acetaldehyde molecule to another, and the subsequent formation of cationic moieties. Density functional theory computations reveal several plausible pathways of proton transfer in mixed cluster cations. Among these pathways, water-mediated proton transfer is energetically favored. Mass spectra and photoionization efficiency curves confirm these theoretical findings and also demonstrate the increased stability of cluster cations where acetaldehyde molecules are symmetrically bonded to the hydronium ion.
O) of one acetaldehyde molecule to another, and the subsequent formation of cationic moieties. Density functional theory computations reveal several plausible pathways of proton transfer in mixed cluster cations. Among these pathways, water-mediated proton transfer is energetically favored. Mass spectra and photoionization efficiency curves confirm these theoretical findings and also demonstrate the increased stability of cluster cations where acetaldehyde molecules are symmetrically bonded to the hydronium ion.


 Please wait while we load your content...
Please wait while we load your content...