Water adsorption and O-defect formation on Fe2O3(0001) surfaces†
Abstract
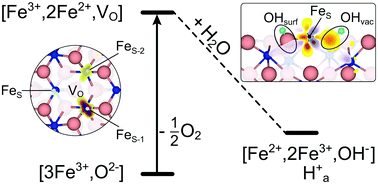
The stability and reactivity of the hematite, Fe2O3(0001) surface are studied by density functional theory including an on-site Coulomb term (DFT+U). Even under oxygen rich conditions, the metal-terminated surface is shown to be stable. On this surface termination, the isolated water molecule forms a heterolytically dissociated structure with the OH− group attached to a surface Fe3+ ion and the proton to a surface O2− ion. Dissociative adsorption is strongly enhanced at oxygen vacancy sites. Here, the OH− group fills the oxygen vacancy site. Dehydrogenation accompanied by defect healing is favoured compared to water desorption (178 kJ mol−1 compared to 236 kJ mol−1). The water adsorption energies (at 0 K) for the clean and defective surfaces are 100 kJ mol−1 and 288 kJ mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...