Estimation of activation energy for electroporation and pore growth rate in liquid crystalline and gel phases of lipid bilayers using molecular dynamics simulations†
Abstract
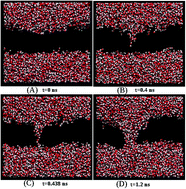
Molecular dynamics simulations of electroporation in POPC and DPPC lipid bilayers have been carried out at different temperatures ranging from 230 K to 350 K for varying electric fields. The dynamics of pore formation, including threshold field, pore initiation time, pore growth rate, and pore closure rate after the field is switched off, was studied in both the gel and liquid crystalline (Lα) phases of the bilayers. Using an Arrhenius model of pore initiation kinetics, the activation energy for pore opening was estimated to be 25.6 kJ mol−1 and 32.6 kJ mol−1 in the Lα phase of POPC and DPPC lipids respectively at a field strength of 0.32 V nm−1. The activation energy decreases to 24.2 kJ mol−1 and 23.7 kJ mol−1 respectively at a higher field strength of 1.1 V nm−1. At temperatures below the melting point, the activation energy in the gel phase of POPC and DPPC increases to 28.8 kJ mol−1 and 34.4 kJ mol−1 respectively at the same field of 1.1 V nm−1. The pore closing time was found to be higher in the gel than in the Lα phase. The pore growth rate increases linearly with temperature and quadratically with field, consistent with viscosity limited growth models.

 Please wait while we load your content...
Please wait while we load your content...