Configurationally stable, enantioenriched organometallic nucleophiles in stereospecific Pd-catalyzed cross-coupling reactions: an alternative approach to asymmetric synthesis
Abstract
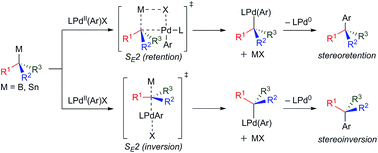
Several research groups have recently developed methods to employ configurationally stable, enantioenriched organometallic nucleophiles in stereospecific Pd-catalyzed cross-coupling reactions. By establishing the absolute configuration of a chiral alkyltin or alkylboron nucleophile prior to its use in cross-coupling reactions, new stereogenic centers may be rapidly and reliably generated with preservation of the known initial stereochemistry. While this area of research is still in its infancy, such stereospecific cross-coupling reactions may emerge as simple, general methods to access diverse, optically active products from common enantioenriched organometallic building blocks. This minireview highlights recent progress towards the development of general, stereospecific Pd-catalyzed cross-coupling reactions using configurationally stable organometallic nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...