Chiral dirhodium catalysts derived from l-serine, l-threonine and l-cysteine: design, synthesis and application†
Abstract
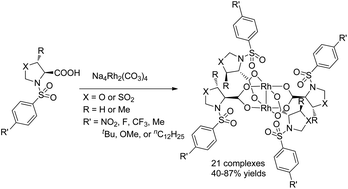
A series of dirhodium tetrakis((4S)-3-(arylsulfonyl)oxazolidine-4-carboxylate), dirhodium tetrakis((4S,5R)-5-methyl-3-(arylsulfonyl)oxazolidine-4-carboxylate) and dirhodium tetrakis((4R)-3-(arylsulfonyl)thiazolidine-4-carboxylate 1,1-dioxide) complexes with different para-substituted arylsulfonyl groups (e.g. –NO2, –F, –CF3, –Me, –tBu, –OMe and –nC12H25) derived from L-serine, L-threonine and L-cysteine, respectively, were prepared with yields in the range of 40–87% through refluxing ligands in water with Na4Rh2(CO3)4. These chiral Rh(II) complexes have been fully characterized by EA, IR, UV-vis, NMR and specific rotation measurements. They are found to be effective chiral catalysts for asymmetric aziridination and cyclopropanation reactions in terms of reactivity and enantioselectivity. They are extremely stable and can be stored for a long period (at least 18 months) on the bench without adversely affecting their reactivity and selectivity. The heterocyclic rings as well as the substituents on the arylsulfonyl groups have critical effects on the degree of asymmetric induction. In general, a higher enantioselectivity was observed in the reactions catalyzed by the oxazolidine-4-carboxylate-derived catalysts than the thiazolidine-4-carboxylate 1,1-dioxide-based catalysts. Among these 21 new Rh(II) catalysts, the uses of dirhodium tetrakis((4S)-3-((4-dodecylphenyl)sulfonyl)oxazolidine-4-carboxylate) (Rh2(4S-DOSO)4) and dirhodium tetrakis((4S,5R)-5-methyl-3-((4-nitrophenyl)sulfonyl)oxazolidine-4-carboxylate) (Rh2(4S,5R-MNOSO)4) resulted in the highest levels of enantioselectivity in aziridination (94% ee) and cyclopropanation (98% ee) of styrene, respectively. The successful design and syntheses of these novel Rh(II) complexes enlarged the scope of accessible chiral dirhodium(II) catalysts.


 Please wait while we load your content...
Please wait while we load your content...