One pot synthesis of diarylfurans from aryl esters and PhI(OAc)2via palladium-associated iodonium ylides†
Abstract
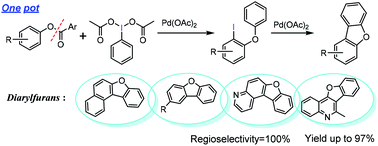
The example of palladium-catalyzed intermolecular cyclization for the synthesis of various diarylfurans in which one of the aromatic rings originates from the phenolic part of the starting ester and the other one from PhI(OAc)2 has been reported. The reaction is carried out through two steps: the rearrangement of palladium-associated iodonium ylides to form o-iodo diaryl ether and then palladium catalyzed intramolecular direct arylation. This reaction can tolerate a variety of functional groups and is alternative or complementary to the previous methods for the synthesis of diarylfurans.

 Please wait while we load your content...
Please wait while we load your content...