Receptor-templated stapling of intrinsically disordered peptide ligands†
Abstract
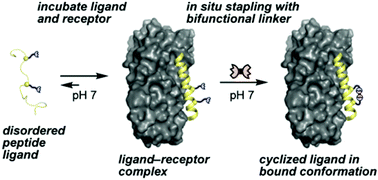
We report here a chemoselective peptide “stapling” method that can be performed on ligand–receptor complexes in situ. An appropriately structured macrocyclic bis-oxime linkage is shown to improve the affinity of a peptide ligand for its native protein receptor. The presence of the receptor as a template to preorganize the ligand into its bioactive conformation is found to bias reaction outcomes, suggesting the potential application of the method for receptor-assisted selection of stapled peptides.

 Please wait while we load your content...
Please wait while we load your content...