Facile synthesis of spirooxindole-pyrazolines and spirobenzofuranone-pyrazolines and their fungicidal activity†
Abstract
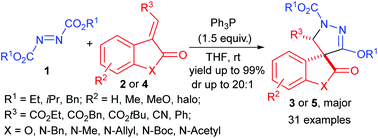
Novel spirooxindole-pyrazolines and spirobenzofuranone-pyrazolines have been synthesized in good to excellent yields via the annulation reactions of the corresponding 3-alkylideneoxindoles and 3-alkylidenebenzofuranones with Huisgen zwitterions. The preliminary bioassay demonstrated that some of the spiropyrazolines possess good in vitro fungicidal activity against several crop fungi at a concentration of 50 μg mL−1.

 Please wait while we load your content...
Please wait while we load your content...