Synthesis of 2-anilinopyridyl–triazole conjugates as antimitotic agents†
Abstract
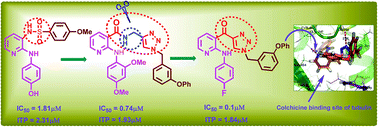
A series of 2-anilinopyridyl–triazole conjugates (6a–t) were prepared and evaluated for their cytotoxic activity against a panel of three human cancer cell lines. Among them compounds 6q, 6r and 6s showed significant cytotoxic activity with IC50 values ranging from 0.1 to 4.1 μM. Structure–activity relationships were elucidated with various substitutions on these conjugates. Flow cytometric analysis revealed that these compounds arrest the cell cycle at the G2/M phase and induce cell death by apoptosis. The tubulin polymerization assay and immunofluorescence analysis showed that these compounds (6q, 6r and 6s) effectively inhibited the microtubule assembly in human prostate cancer cells (DU-145). The docking studies showed that 6s interacts and binds efficiently with the tubulin protein at the colchicine binding site. This was further confirmed by the colchicine competitive binding assay. Moreover, compounds 6q, 6r and 6s possess anti-tubulin activity both in vitro and within cells as demonstrated by the ratio of soluble versus polymerized tubulin. Further the apoptotic effects of compounds were confirmed by Hoechst staining, caspase 3 activation, annexin-V FITC, mitochondrial membrane potential and DNA fragmentation analysis. Interestingly, these compounds did not affect the normal human embryonic kidney cells, HEK-293.

 Please wait while we load your content...
Please wait while we load your content...