Pd-catalyzed asymmetric allylic amination using easily accessible metallocenyl P,N-ligands†
Abstract
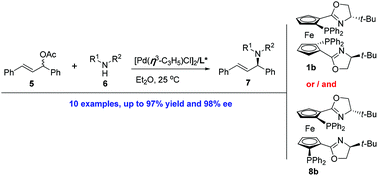
Compared to their C1-symmetric counterparts, planar chiral C2-symmetric metallocenyl P,N-ligands are efficient chiral ligands for Pd-catalyzed asymmetric allylic aminations, providing a number of amination products with high enantioselectivities. A non-C2-symmetric ferrocenyl P,N-ligand (a by-product obtained during the synthesis of the above C2-symmetric species) was also found to be an efficient ligand for asymmetric allylic aminations. A mixed ligand system consisting of both C2- and non-C2-symmetric ferrocene complexes was examined and showed high catalytic activity with the amination products being obtained with excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...