The synthesis of head-to-tail cyclic sulfono-γ-AApeptides†
Abstract
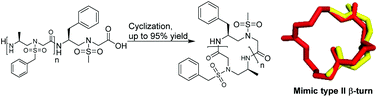
We report an efficient method for the preparation of unprecedented head-to-tail cyclic sulfono-γ-AApeptides. Following this method, a number of cyclic sequences bearing two to five subunits were efficiently synthesized. In addition, the X-ray crystal structure study of a three-membered cyclic sulfono-γ-AApeptide revealed a type II β-turn-like character.
 Please wait while we load your content...
Please wait while we load your content...