Intramolecular pyridine-based frustrated Lewis-pairs†
Abstract
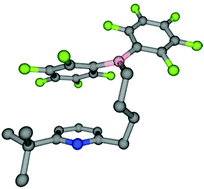
Deprotonation of the methylpyridines 2,6-lutidine, 2-picoline, 4-dimethylamino-2,6-dimethylpyridine as well as 2,6-dimethyl-4-(piperidine-1-yl)pyridine with n-butyllithium or n-butyllithium/KO-t-Bu at the methyl positions led to the corresponding organolithium or -potassium compounds. Treatment with ClB(C6F5)2 resulted in formation of the 2-borylmethylpyridines py-CH2-B(C6F5)2. They are monomeric and form intramolecular B–N bonds and four-membered rings. A short intramolecular B–N distance was observed in the crystal structure of the dimethylamino-functionalized derivative and proposed to be responsible for the low reactivity of the products towards hydrogen, thf, acetonitrile and CO2. Hydroboration of 6-tert-butyl-2-but-4′-enylpyridine with HB(C6F5)2 led to the corresponding hydroboration product t-Bu-py-(CH2)4-B(C6F5)2 which shows no intramolecular B–N bond formation due to steric crowding. H/D-scrambling experiments with a H2/D2 mixture revealed its reactivity towards hydrogen.

 Please wait while we load your content...
Please wait while we load your content...