Thermal evolution of the crystal structure of proton conducting BaCe0.8Y0.2O3−δ from high-resolution neutron diffraction in dry and humid atmosphere
Abstract
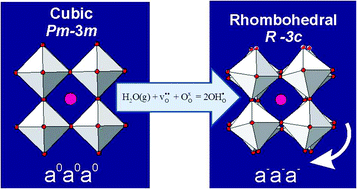
The crystal structure of the proton conducting perovskite BaCe0.8Y0.2O3−δ (BCY20) has been studied via high-resolution in situ neutron diffraction performed in controlled dry and humid (heavy water) oxygen flow. Two phase transitions, cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m→R
m→R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (775 °C)→Imma (250 °C) were observed on cooling from 1000 °C in dry O2. A significant shift of the phase stability fields was observed on cooling in wet oxygen (pD2O ≈ 0.2 atm) with the R
c (775 °C)→Imma (250 °C) were observed on cooling from 1000 °C in dry O2. A significant shift of the phase stability fields was observed on cooling in wet oxygen (pD2O ≈ 0.2 atm) with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c structure stabilised at 900 °C, and the R
c structure stabilised at 900 °C, and the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c→Imma transition occurring at 675 °C. On cooling below 400 °C a monoclinic, I2/m, phase started to appear. The structural dependence on hydration level is primarily due to the de-stabilisation of the correlated, octahedra tilts as a consequence of structural relaxation around the oxygen vacancies present in the non-hydrated phase. The tendency of hydrated BaCe0.8Y0.2O3−δ to show octahedral tilting is also found to be enhanced, indicating that the deuteronic (protonic) defects influence the crystal structure, possibly via hydrogen bonding. Stabilisation of the monoclinic I2/m phase is attributed to the structural effect of deuterons that is inferred to increase on cooling as deuterons localise to a greater extent. Changing from wet oxidising (O2 + D2O(g)) to wet reducing (5% H2 in Ar + D2O(g)) atmosphere did not influence the structure or the phase stability, indicating that Ce4+ was not reduced under the present conditions. Based on the observed cell volume expansion protonic defects are present in the material at 900 °C at a D2O partial pressure of ∼0.2 atm. The origin of the chemical expansion is explained by the effective size of the oxygen vacancy being significantly smaller than the [OD] defect. Rietveld analysis has been used to locate possible sites for the deuterons in the high temperature, R
c→Imma transition occurring at 675 °C. On cooling below 400 °C a monoclinic, I2/m, phase started to appear. The structural dependence on hydration level is primarily due to the de-stabilisation of the correlated, octahedra tilts as a consequence of structural relaxation around the oxygen vacancies present in the non-hydrated phase. The tendency of hydrated BaCe0.8Y0.2O3−δ to show octahedral tilting is also found to be enhanced, indicating that the deuteronic (protonic) defects influence the crystal structure, possibly via hydrogen bonding. Stabilisation of the monoclinic I2/m phase is attributed to the structural effect of deuterons that is inferred to increase on cooling as deuterons localise to a greater extent. Changing from wet oxidising (O2 + D2O(g)) to wet reducing (5% H2 in Ar + D2O(g)) atmosphere did not influence the structure or the phase stability, indicating that Ce4+ was not reduced under the present conditions. Based on the observed cell volume expansion protonic defects are present in the material at 900 °C at a D2O partial pressure of ∼0.2 atm. The origin of the chemical expansion is explained by the effective size of the oxygen vacancy being significantly smaller than the [OD] defect. Rietveld analysis has been used to locate possible sites for the deuterons in the high temperature, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c and Imma, phases that are most relevant for proton transport.
c and Imma, phases that are most relevant for proton transport.
- This article is part of the themed collection: Perovskites

 Please wait while we load your content...
Please wait while we load your content...