Chemical modifications and stability of phosphorene with impurities: a first principles study
Abstract
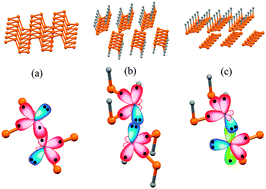
We perform a systematic first-principles study of phosphorene in the presence of typical monovalent (hydrogen and fluorine) and divalent (oxygen) impurities. The results of our modeling suggest a decomposition of phosphorene into weakly bonded one-dimensional (1D) chains upon single- and double-side hydrogenation and fluorination. In spite of a sizable quasiparticle band gap (2.29 eV), fully hydrogenated phosphorene was found to be dynamically unstable. In contrast, complete fluorination of phosphorene gives rise to a stable structure, which is an indirect gap semiconductor with a band gap of 2.27 eV. We also show that fluorination of phosphorene from the gas phase is significantly more likely than hydrogenation due to the relatively low energy barrier for the dissociative adsorption of F2 (0.19 eV) compared to H2 (2.54 eV). At low concentrations, monovalent impurities tend to form regular atomic rows of phosphorene, though such patterns do not seem to be easily achievable due to high migration barriers (1.09 and 2.81 eV for H2 and F2, respectively). Oxidation of phosphorene is shown to be a qualitatively different process. Particularly, we observe instability of phosphorene upon oxidation, leading to the formation of disordered amorphous-like structures at high concentrations of impurities.

 Please wait while we load your content...
Please wait while we load your content...