Enantioselective synthesis of fused heterocycles with contiguous stereogenic centers by chiral phosphoric acid catalyzed symmetry breaking†
Abstract
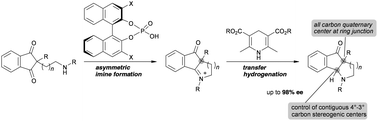
Described herein is a highly enantioselective synthesis of fused piperidine and pyrrolidine derivatives with all-carbon stereogenic centers. The enantioselective reductive amination from Cs-symmetric 1,3-dione derivatives proceeded in a highly stereoselective manner by taking advantage of the desymmetrization approach to afford fused heterocycles with contiguous stereogenic centers in good to excellent enantioselectivities (up to 98% ee).

 Please wait while we load your content...
Please wait while we load your content...