An innate immune system-mimicking, real-time biosensing of infectious bacteria†
Abstract
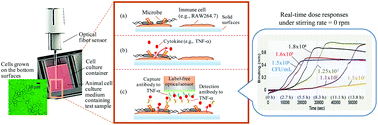
An animal cell-based biosensor was investigated to monitor bacterial contamination in an unattended manner by mimicking the innate immune response. The cells (RAW 264.7 cell line) were first attached onto the solid surfaces of a 96-well microtiter plate and co-incubated in the culture medium with a sample that might contain bacterial contaminants. As Toll-like receptors were present on the cell membrane surfaces, they acted as a sentinel by binding to pathogen-associated molecular patterns (PAMPs) of any contaminant. Such biological recognition initiates signal transmission along various pathways to produce different proinflammatory mediators, one of which, tumor necrosis factor-α (TNF-α) was measured using an immunosensor. To demonstrate automated bacterium monitoring, a capture antibody specific for TNF-α was immobilized on an optical fiber sensor tip and then used to measure complex formation in a label-free sensor system (e.g., Octet Red). The sensor response time depended significantly on the degree of agitation of the culture medium, controlling the biological recognition and further autocrine/paracrine signaling by cytokines. The response, particularly under non-agitated conditions, was also influenced by the medium volume, revealing a local gradient change of the cytokine concentration and also acidity, caused by bacterial growth near the bottom surfaces. A biosensor system retaining 50 μL medium and not employing agitation could be used for the early detection of bacterial contamination. This novel biosensing model was applied to the real-time monitoring of different bacteria, Shigella sonnei, Staphylococcus aureus, and Listeria monocytogenes. They (<100 CFU mL−1) could be detected automatically within the working time. Such analysis was carried out without any manual handling regardless of the bacterial species, suggesting the concept of non-targeted bacterial real-time monitoring. This technique was further applied to real sample testing (e.g., with milk) to exemplify, for example, the food quality control process without using any additional sample pretreatment such as magnetic concentration.

 Please wait while we load your content...
Please wait while we load your content...