Conformational dynamics of α-synuclein: insights from mass spectrometry†
Abstract
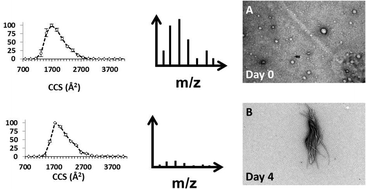
The aggregation and deposition of α-synuclein in Lewy bodies is associated with the progression of Parkinson's disease. Here, Mass Spectrometry (MS) is used in combination with Ion Mobility (IM), chemical crosslinking and Electron Capture Dissociation (ECD) to probe transient structural elements of α-synuclein and its oligomers. Each of these reveals different aspects of the conformational heterogeneity of this 14 kDa protein. IM-MS analysis indicates that this protein is highly disordered, presenting in positive ionisation mode with a charge state range of 5 ≤ z ≤ 21 for the monomer, along with a collision cross section range of ∼1600 Å2. Chemical crosslinking applied in conjunction with IM-MS captures solution phase conformational families enabling comparison with those exhibited in the gas phase. Crosslinking IM-MS identifies 3 distinct conformational families, Compact (∼1200 Å2), Extended (∼1500 Å2) and Unfolded (∼2350 Å2) which correlate with those observed in solution. ECD-Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry (ECD-FT-ICR MS) highlights the effect of pH on α-synuclein structure, identifying the conformational flexibility of the N and C termini as well as providing evidence for structure in the core and at times the C terminus. A hypothesis is proposed for the variability displayed in the structural rearrangement of α-synuclein following changes in solution pH. Following a 120 h aggregation time course, we observe an increase in the ratio of dimer to monomer, but no gross conformational changes in either, beyond the significant variations that are observed day-to-day from this conformationally dynamic protein.
- This article is part of the themed collection: Analytical Sciences in the UK

 Please wait while we load your content...
Please wait while we load your content...