Ozonation of methylenecyclopropanes†
Abstract
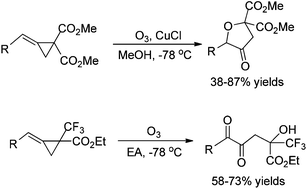
Ozonation of methylenecyclopropanes bearing gem-disubstituted electron-withdrawing groups (EWG) gave ring-opened oxidative products in moderate to good yields. For MCPs in which EWGs are two methoxycarbonyl groups, the ozonation gave oxidative cyclization products in methanol at −78 °C in the presence of CuCl; for MCPs in which EWGs are one methoxycarbonyl and one trifluoromethyl group, the ozonation produced α-diketones in ethyl acetate (EA) at −78 °C.

 Please wait while we load your content...
Please wait while we load your content...