Micellar control of the photooxidation pathways of 10-methyl phenothiazine: electron versus energy transfer mechanisms†‡§
Abstract
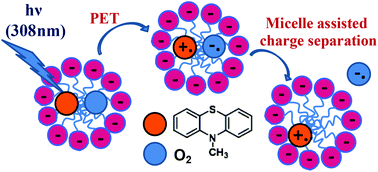
10-Methyl phenothiazine (MPS) was chosen as a model compound to investigate the effects of compartmentalisation and of charged interfaces on the primary mechanisms involved in the phototoxic reactions related to phenothiazine drugs. Two most important pathways resulting from the interaction of the triplet excited state of MPS (3MPS*) with molecular oxygen (3O2) have to be considered: (i) energy transfer producing singlet oxygen (1O2) and (ii) electron transfer generating the superoxide anion (O2˙−) and the radical cation (MPS˙+). The quantum yields of 1O2 production by MPS solubilized in the dispersed pseudo-phase of aqueous micellar systems were found to be similar to those determined in solvents of various polarities, regardless of the anionic or cationic nature of the surfactant (SDS or CTAC). However, micellar compartmentalisation and surfactant charge affect considerably both the sensitized and the self-sensitized photooxidation of MPS. The formation of 10-methyl phenothiazine sulfoxide (MPSO), produced by the reaction of MPS with 1O2, proceeds at a higher rate in SDS micelles than in neat polar solvents. This result may be explained by the protonation of the zwitterionic intermediate Z (MPS+OO−) at the micellar interface to yield the corresponding cation C (MPS+OOH) that is stabilized in the negatively charged micelles and reacts much faster with MPS than Z to yield MPSO. The electron transfer reaction from 3MPS* to O2 yielding MPS˙+ and O2˙− is also enhanced in SDS micelles, as back electron transfer (BET) is prevented by ejection of O2˙− to the aqueous bulk phase and stabilization of MPS˙+ in the anionic micelles. The size of the SDS micelles modulates the relative contribution of each pathway (formation of MPSO or MPS˙+) to the overall conversion of MPS to its oxidation products. Photooxidation of MPS in cationic micelles is a very slow process, as the formation of neither C nor MPS˙+ is favoured in positively charged micelles.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...