Asymmetric synthesis of substituted NH-piperidines from chiral amines†
Abstract
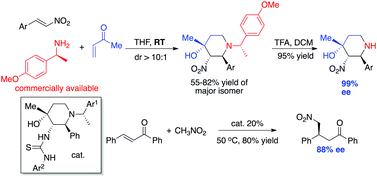
Previously, we reported an efficient asymmetric synthesis of substituted piperidines through an exocyclic chirality induced nitroalkene/amine/enone (NAE) condensation reaction. An effective protecting group strategy was developed herein to achieve enantiopure piperidines (yields up to 92%) with complete chirality retention (ee > 95%). A simple derivatization of the obtained piperidines gave thiourea catalysts, indicating the strong potential of this method for producing new amine-based dual functional organocatalysts for future development.

 Please wait while we load your content...
Please wait while we load your content...