Synthesis, characterization, photophysical properties, and catalytic activity of an SCS bis(N-heterocyclic thione) (SCS-NHT) Pd pincer complex†
Abstract
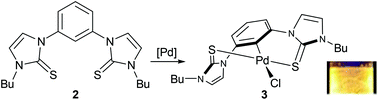
Treatment of 1,3-bis(3′-butylimidazolyl-1′-yl)benzene diiodide with elemental sulfur in the presence of a base produced a bis(N-heterocyclic thione) (NHT) pincer ligand precursor. Its reaction with PdCl2(CH3CN)2 produced chloro[1,3-bis(3′-butylimidazole-2′-thione-κ-S)benzene-κ-C]palladium(II), a 6,6-fused ring SCS-NHT palladium pincer complex. This air stable compound is, to our knowledge, the first SCS pincer complex that utilizes N-heterocyclic thione (NHT) donor groups. The molecular structures of the ligand precursor and the palladium complex were determined by X-ray crystallography and computational studies provided insight into the interconversion between its rac and meso conformations. The photophysical properties of the complex were established, and its catalytic activity in Suzuki, Heck, and Sonogashira cross-coupling reactions was evaluated.

 Please wait while we load your content...
Please wait while we load your content...