Ferrocene–isocoumarin conjugated molecules: synthesis, structural characterization, electronic properties, and DFT–TDDFT computational study†
Abstract
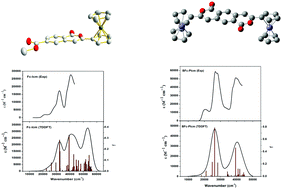
Two ferrocene–isocoumarin conjugated molecules, methyl 3-ferrocenyl-1-oxo-1H-isochromene-6-carboxylate (Fc-Icm) and 3,8-bisferrocenylpyrano[3,4-g]isochromene-1,6-dione (BFc-PIcm), have been synthesized through the acid-prompted regioselective oxidative cyclization from dimethyl 2-(ferrocenylethynyl)terephthalate (Fc-TP) and dimethyl 2,5-bis(ferrocenylethynyl)terephthalate (BFc-TP), respectively. Single-crystal X-ray diffraction, together with the density functional theory (DFT) calculations, shows that the ferrocene–isocoumarin conjugated compounds display better coplanarity than the corresponding ferrocenylethynyl terephthalates. All the compounds exhibit characteristic MLCT, ICT and π–π* transitions in the UV-visible range in solution, and Fc-Icm and BFc-PIcm show higher oscillator strength of the absorption than Fc-TP and BFc-TP, which are verified by time-dependent DFT (TDDFT) theoretical calculations. The electrochemical properties are studied by cyclic voltammetry (CV), which are also in accord with the theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...