Abstract
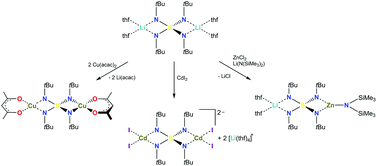
Three novel metal complexes [(acac)2Cu2(NtBu)4S] (3), [Li(thf)4]2[I4Cd2(NtBu)4S] (4) and [(thf)2Li{(SiMe3)2N}Zn(NtBu)4S] (5) are prepared from the intended transmetalation of the dilithium complex of N,N′,N′′,N′′′-tetrakis(tert-butyl)tetraimidosulfate [(thf)4Li2(NtBu)4S] (1). The two lithium cations are replaced by either the cationic (acac)Cu(II) moiety, the neutral I2Cd(II) residue or only a single lithium cation is substituted by the cationic (Me3Si)2NZn(II) fragment. The complexes show two main results: first the S(NtBu)42− tetrahedron can serve as a ligand to transition metals from the soft Cu(II) to the harder Zn(II) at opposite sides and second the S–N bond distances vary only marginally in response to the various metals and the four distances constantly sum up to 6.38(2) Å. Hence the electropositive sulfur atom responds by internal shift to the metal-polarized negative charge at the outside of the S(NR)42− tetrahedron.

 Please wait while we load your content...
Please wait while we load your content...