Thermodynamics for complex formation between palladium(ii) and oxalate†
Abstract
Complex formation between [Pd(H2O)4]2+ and oxalate (ox = C2O42−) has been studied spectrophoto-metrically in aqueous solution at variable temperature, ionic strength and pH. Thermodynamic parameters at 298.2 K and 1.00 mol dm−3 HClO4 ionic medium for the complex formation [Pd(H2O)4]2+ + H2ox ⇄ [Pd(H2O)2(ox)] + 2H3O+ with equilibrium constant K1,H (in mol dm−3) are log10K1,H = 3.38 ± 0.08, ΔH01 = −33 ± 3 kJ mol−1, and ΔS01 = −48 ± 11 J K−1 mol−1, as determined from spectrophotometric equilibrium titrations at 15.0, 20.0, 25.0 and 31.0 °C. Thermodynamic overall stability constants β0n (in (mol dm−3)−n, n = 1,2) for [Pd(H2O)2(ox)] and [Pd(ox)2]2− at zero ionic strength and 298.2 K, defined as the equilibrium constants for the reaction Pd2+ + nox2− ⇄ [Pd(ox)n]2−2n (water molecules omitted) are log10β01 = 9.04 ± 0.06 and log10β02 = 13.1 ± 0.3, respectively, calculated by use of Specific Ion Interaction Theory from spectrophotometric titrations with initial hydrogen ion concentrations of 1.00, 0.100 and 0.0100 mol dm−3 and ionic strengths of 1.00, 2.00 or 3.00 mol dm−3. The values derived together with literature data give estimated overall stability constants for Pd(II) compounds such as [Pd(en)(ox)] and cis-[Pd(NH3)2Cl2], some of them analogs to Pt(II) complexes used in cancer treatment. The palladium oxalato complexes are significantly more stable than palladium(II) complexes with monodentate O-bonding ligands. A comparison between several different palladium complexes shows that different parameters contribute to the stability variations observed. These are discussed together with the so-called chelate effect.
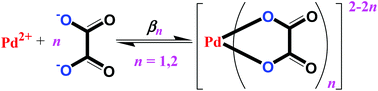

 Please wait while we load your content...
Please wait while we load your content...