Directional properties of fluorenylidene moieties in unsymmetrically substituted N-heterocyclic carbenes. Unexpected CH activation of a methylfluorenyl group with palladium. Use in palladium catalysed Suzuki–Miyaura cross coupling of aryl chlorides†
Abstract
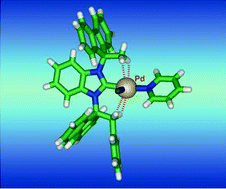
Benzimidazolium salts having their two nitrogen atoms substituted by different 9-alkylfluorenyl groups (4a–e and 4g, alkyl1/alkyl2 = Me/Et, Me/Pr, Me/n-Bu, Me/i-Pr, Me/Bn, Me/CH2SMe have been synthesised in high yields in two or three steps from N,N′-bis(9H-fluoren-9-ylidene)benzene-1,2-diamine (1). The imidazolium salts 4a–e were converted readily into the corresponding PEPPSI-type palladium complexes (PEPPSI = pyridine-enhanced precatalyst preparation stabilisation and initiation), while reaction of the methylthioether-substituted salt 4g with PdCl2/K2CO3/pyridine afforded the palladacycle 5g resulting from metallation of the methyl group attached to the fluorenylidene moiety. NMR and X-ray diffraction studies revealed that the carbene ligands in 5a–5e behave as clamp-like ligands, the resulting metal confinement arising from a combination of the orientational properties of the fluorenylidene moieties that push the alkyl groups towards the metal centre and attractive anagostic interactions involving CH2(fluorenyl) groups. Complexes 5a–e were assessed in Suzuki–Miyaura cross-coupling reactions. Like their symmetrical analogues they displayed high activity in the coupling of phenyl boronic acid with p-tolylchloride but their performance remained slightly inferior to that of the related, symmetrical Et/Et complex 5h.

 Please wait while we load your content...
Please wait while we load your content...