Highly effective design strategy for the heterogenisation of chemo- and enantioselective organocatalysts†
Abstract
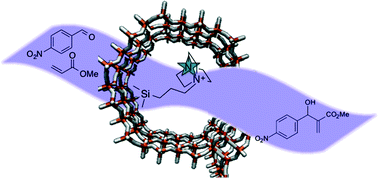
We have demonstrated that the covalent heterogenisation of two homogeneous organocatalysts, cinchonine and 1,4-diazabicyclo[2.2.2]octane, onto the inner walls of mesoporous silica supports results in highly active and selective solid catalysts that are easily recoverable and recyclable. We have further highlighted the efficacy of our design rationale and its amenability for tailoring the nature of the active site via meticulous choice of pore-aperture and hydrophobicity to create a superior heterogenised analogue for Michael addition and Baylis Hillman reactions. It is envisaged that this immobilisation strategy could be rationally extended to the heterogenisation of a plethora of organocatalysts.
- This article is part of the themed collection: Catalysis on Chiral Surfaces: From Fundamental Aspects to Application


 Please wait while we load your content...
Please wait while we load your content...