Photo-active and dynamical properties of hematite (Fe2O3)–water interfaces: an experimental and theoretical study†
Abstract
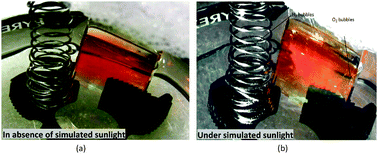
The dynamical properties of physically and chemically adsorbed water molecules at pristine hematite-(001) surfaces have been studied by means of equilibrium Born–Oppenheimer molecular dynamics (BOMD) in the NVT ensemble at 298 K. The dissociation of water molecules to form chemically adsorbed species was scrutinised, in addition to ‘hopping’ or swapping events of protons between water molecules. Particular foci have been dynamical properties of the adsorbed water molecules and OH− and H3O+ ions, the hydrogen bonds between protons in water molecules and the bridging oxygen atoms at the hematite surface, as well as the interactions between oxygen atoms in adsorbed water molecules and iron atoms at the hematite surface. Experimental results for photoelectrical current generation complement simulation findings of water dissociation.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...