Non-covalent interactions at electrochemical interfaces: one model fits all?
Abstract
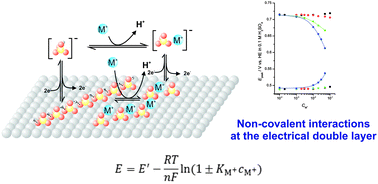
The shift with increasing concentration of alkali-metal cations of the potentials of both the spike and the hump observed in the cyclic voltammograms of Pt(111) electrodes in sulfuric acid solutions is shown to obey the simple model recently developed by us to explain the effect of non-covalent interactions at the electrical double layer. The results suggest that the model, originally developed to describe the effect of alkali-metal cations on the cyclic voltammogram of cyanide-modified Pt(111) electrodes, is of general applicability and can explain quantitatively the effect of cations on the properties of the electrical double layer.

 Please wait while we load your content...
Please wait while we load your content...