A DFT study on carbon monoxide adsorption onto hydroxylated α-Al2O3(0001) surfaces†
Abstract
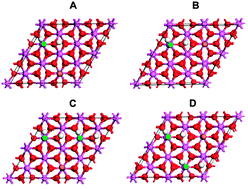
The adsorption of CO onto the hydroxylated α-Al2O3(0001) surface was studied using density functional theory (DFT). Dissociated adsorption of water was found to be stable, with an adsorption energy (Ea) of 1.62 eV at θwater = 0.75. The most stable hydroxylation form on the clean surface was found to be in the 1–2 dissociation configuration, where the OH group binds to a surface Al ion and the H ion binds to one of the three equivalent surface O ions. The adsorption energy of CO was found to be dependent on the degree of pre-hydroxylation of the surface as well as on the CO coverage. The highest adsorption energy of CO was found when θCO = 0.25 on a pre-hydroxylated surface with θwater = 0.25; Ea = 0.57 eV. The adsorption energy of CO decreased upon increasing the degree of pre-hydroxylation. The vibrational frequency of νCO was also computed and in all cases it was blue shifted with respect to gas-phase CO. The shift, Δν, decreased with increasing CO coverage but increased with increasing surface hydroxylation. A comparison with available experimental work is discussed.

 Please wait while we load your content...
Please wait while we load your content...