Phase transitions in θ-(ET)4MnBr4(C6H6−nCln) (n = 1, 2) driven by ordering in anion and/or cation layers†
Abstract
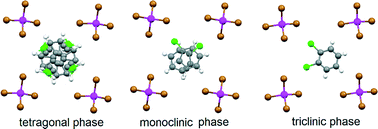
Newly synthesized layered organic conductors θ-(ET)4MnBr4(1,2-C6H4Cl2) (1) and θ-(ET)4MnBr4(C6H5Cl) (2) show metal-like conductivity along conducting layers down to 4.3 and 60 K, respectively, while showing semiconducting properties in the perpendicular direction. Phase transitions driven by ordering in anion layers in 1 and ordering in a half of cation layers in 2 were found to occur at 297 and ~240 K, respectively. Crystal structures above and below the phase transitions were determined for 1 at 260 and 333 K and for 2 at 260 and 220 K. In the above-transition phases of both compounds, attributed to the tetragonal crystal system, all ET layers are similar in structure. In the below-transition phases, attributed to triclinic and monoclinic crystal systems, the neighboring ET layers of each phase differ in periods along the stacks and in dihedral angles θ between radical cations of the adjacent stacks.

 Please wait while we load your content...
Please wait while we load your content...