Morphological and orientational diversity of LiFePO4 crystallites: remarkable reaction path dependence in hydrothermal/solvothermal syntheses†
Abstract
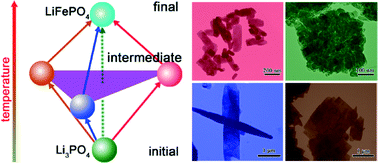
We design four-group experiments to understand the morphological and orientational diversity of LiFePO4 crystallites in hydrothermal and/or solvothermal syntheses. In the solvothermal synthesis, in which water is highly deficient, the starting Li3PO4 nanoparticle likely directly evolves into LiFePO4 through an in situ transition mechanism. In contrast, under the other three conditions, i.e., hydrothermal synthesis with stoichiometric H3PO4, hydrothermal and solvothermal syntheses in the presence of excess H3PO4, the starting Li3PO4 nanoparticle follows three diverse paths, generating different precursors and/or intermediates whose compositions and dissolution properties are remarkably divergent. Such divergences in reaction paths dramatically influence the colloidal stability of the small, primary nanosheets participating in oriented-attachment aggregation growth, resulting in the diversity of the resultant LiFePO4 in crystallite size from nanometer to micrometer shape (rod-like platelet, slab, and flake), and orientation ([010], [100] and [211]) as well as point defect concentration. Electrochemical performances of the diverse LiFePO4 crystallites synthesized in this study correlate well with the morphological and orientational diversity, shedding light on the tailored synthesis of LiFePO4 crystallites for high-performance lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...