Controlling the aspect ratio and electrocatalytic properties of nickel cobaltite nanorods†
Abstract
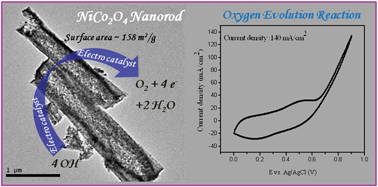
NiCo2O4 nanorods (assembled with small nanoparticles) with different aspect ratios were synthesized through a surfactant mediated reverse micellar route. Anisotropy of the nickel cobaltite nanorods was retained after calcination of nickel cobalt oxalate precursors. A high aspect ratio (∼12) of the NiCo2O4 nanorods was obtained by using a cationic surfactant (CTAB) while a non-ionic surfactant (Tergitol) led to the formation of a much lower aspect ratio (∼5) of the nanorods. These nanorods could potentially be used as anodic electrocatalysts for the oxygen evolution reaction. Due to having a high surface roughness, nanorods assembled with the smallest particles, ∼5–10 nm in diameter (surface area ∼158 m2 g−1), show a high current density of ∼140 mA cm−2 with a low onset potential of ∼0.31 V (at 0.9 V) towards the oxygen evolution reaction and the values of current density is significantly higher than earlier reports.

 Please wait while we load your content...
Please wait while we load your content...